Separating chemical mixtures is an essential step in many chemical production and related industrial processes. Distillation—one of the most common methods for separating chemicals—uses heat to separate chemicals based on their relative volatilities. When the mixture is heated the countercurrent flow of the liquid and vapor establishes a concentration gradient in the distillation column. Depending on the number of chemical compounds in the mixture the process of distillation will often require multiple units to achieve the process goals.
The basic graphical design methodology for a 2 component distillation system was developed in 1925 by McCabe & Thiele. This process is taught to all chemical engineering students and provides a foundational understanding of distillation design and the challenges an engineer will have to overcome to meet the process specifications of a distillation design. Today, the McCabe Thiele diagram has been supplanted by computer simulation programs that can solve large matrices of equations related to many components in the feed stream to a distillation system.
The McCabe-Thiele Diagram
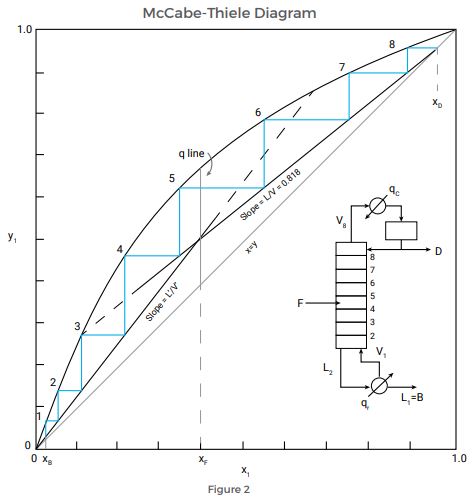 The McCabe-Thiele Diagram is a graphical design procedure used to calculate a distillation system and its operating parameters. A plot of the equilibrium curve of a binary mixture—which displays the mole fraction of a chemical mixture in the liquid phase that is in an equilibrium condition with that chemical mixture in the vapor phase—is the basis of the graphical method.
The McCabe-Thiele Diagram is a graphical design procedure used to calculate a distillation system and its operating parameters. A plot of the equilibrium curve of a binary mixture—which displays the mole fraction of a chemical mixture in the liquid phase that is in an equilibrium condition with that chemical mixture in the vapor phase—is the basis of the graphical method.
The output from the diagram will display the number of theoretical stages, or trays, needed to distill a binary mixture. While the analysis involves several assumptions, these assumptions are generally not significant to the calculation. To determine the number of distillation stages, users plot three lines on top of the equilibrium curve:
- The rectifying section operating line
- The feed line
- The stripping section operating line
Once the operating lines are in place, the stages required are then drawn as right triangles in the space between the equilibrium curve and the lines. The number of triangles equals the number of necessary distillation stages.
Advantages
The McCabe-Thiele method is an excellent educational tool but is rarely used in designing a system in today’s world. The real value of the method is the simplicity it lends to the understanding of the distillation design process.
Computational (Simulation) Methods for Distillation Design
Process-simulation programs have become the main design method for chemical engineers since the development of the PC. Two of the most popular software solutions—Aspen and ChemCad—are great tools that produce accurate solutions. An accurate solution does require that the user of the software has a full understanding of the thermodynamics and component interaction parameters used in the mathematics producing a converged solution in the software package. The notes below detail the limitations and risks of relying on a simulation package as a design basis to build a system.
Advantages
Programs can predict non-ideal behaviors. A process simulator uses different mathematical models to predict both physical and chemical interactions. For example, different models can be used for highly dilute systems, systems with polar components, and systems with non-polar components. An engineer using a process simulator need only choose the correct model to fit different interactions best. That model selection process is a critical step in properly setting up a process simulation.
Combined with comparisons to empirical vapor-liquid equilibrium data, these simulations can closely predict behaviors and identify the ideal distillation process.
Disadvantages
Unfortunately, even process-simulation programs can’t capture every interaction accurately. Some distillation processes will require laboratory tests to determine the right design. This is typically experienced in designs with multiple components that produce conflicting results in a simulation due to the lack of information in the software for chemical interactions.
A Case for Laboratory and Pilot Testing
 Mixtures with multiple components, including interacting solids, and newly developed chemicals will need to undergo laboratory tests.
Mixtures with multiple components, including interacting solids, and newly developed chemicals will need to undergo laboratory tests.
If your company is processing chemical mixtures that have complex interactions or include components that cannot be accurately simulated, Thermal Kinetics can help assist with the design and execution of a pilot testing campaign. The output dataset of a pilot campaign will include the required operating points and sizing information to properly design a full scale operating distillation system that meets the separation process specifications.
Following a pilot test campaign, the results can be added to a process simulation program to regress the data into vapor/liquid equilibrium data. Future simulations can then be used with confidence to predict the full-plant performance. This is a great tool used by many plants when slight changes in operating conditions or composition need evaluation.
The engineers at Thermal Kinetics can help scale up your distillation process or optimize your current process and equipment. Contact Thermal Kinetics today to receive a quote for our process design and pilot plant testing services.
Tags: distillation, distillation process


